Disease of the Month - Duchenne Muscular Dystrophy (DMD)
Shots:
- The Disease of the Month report by PharmaShots aims to integrate an advanced approach to disease analysis. The report delves deep into the epidemiology, market size, disease management, available therapies, and key players involved
- For the April edition of the Disease of the Month report, PharmaShots brings an enlightening guide to Duchenne Muscular Dystrophy, a genetic problem that causes muscles to weaken and atrophy
- The condition most commonly affects children between 3 and 6 years of age
Introduction1,2,3,4,5
Duchenne muscular dystrophy is an inherited disorder that results from a genetic mutation in the dystrophin gene, causing muscle weakness and atrophy. It’s a multi-systemic condition that affects many parts of the body resulting in deterioration of the skeletal, heart, and lung muscles. As the dystrophin gene is found on the X-chromosome, it primarily affects males, while females are typically carriers.
Symptoms1,2,3,4,5
Duchenne muscular dystrophy (DMD) is a genetic disorder characterized by progressive muscle degeneration and weakness, typically onset in early childhood and worsening over time. some common symptoms of DMD include:
- Muscle weakness
- Delayed motor milestones
- Progressive muscle wasting
- Difficulty with motor skills
- Enlarged calves
- Contractures
- Respiratory difficulties
- Heart problems
- Learning difficulties
- Cardiomyopathy
- Delayed speech and language development
- Scoliosis (spine curvature)
- Short stature (height)
Diagnosis1,2,3,4,5
The diagnosis of DMD typically involves a combination of clinical evaluation, genetic testing, and sometimes other diagnostic tests. including:
-
Clinical Evaluation: A comprehensive medical history and physical examination usually precede the diagnosing process, including muscle weakness, delayed motor milestones, difficulty walking or running, enlarged calves, and other characteristic features
-
Genetic Testing: Genetic testing is typically used to confirm the diagnosis of DMD. This usually involves a blood test or a cheek swab to analyze DNA for mutations in the DMD gene. Genetic testing can identify specific mutations in the DMD gene, which can help confirm the diagnosis and provide information about disease severity and prognosis
-
Other Diagnostic Tests: Additional tests may be ordered to assess muscle function, including:
- Creatine kinase (CK) blood test
- Electromyography (EMG) and nerve conduction studies
- Electrocardiogram (EKG)
Early diagnosis of DMD is essential for implementing appropriate medical management, supportive care, and interventions to help improve outcomes and quality of life for affected individuals. Therefore, prompt recognition and evaluation of symptoms are crucial, followed by confirmatory genetic testing for a definitive diagnosis.
Epidemiology6,7
According to the Muscular dystrophy association report, in Europe and North America, the prevalence of Duchenne muscular dystrophy is approx. 6 per 100,000 individuals.
According to the rare disease advisor report, DMD with a prevalence of 1.7 and 4.2 per 100,000. According to the distribution across region, the prevalence of DMD per 100,000 people was 1.7 in Canada, 1.2 in Russia, 5.7 in Japan, 7.7 in Egypt, 1 in South Africa, 6 in Libya, between 1.7 and 3.4 in Italy, 2.9 in Slovenia, and between 3.5 and 4.3 in the UK.
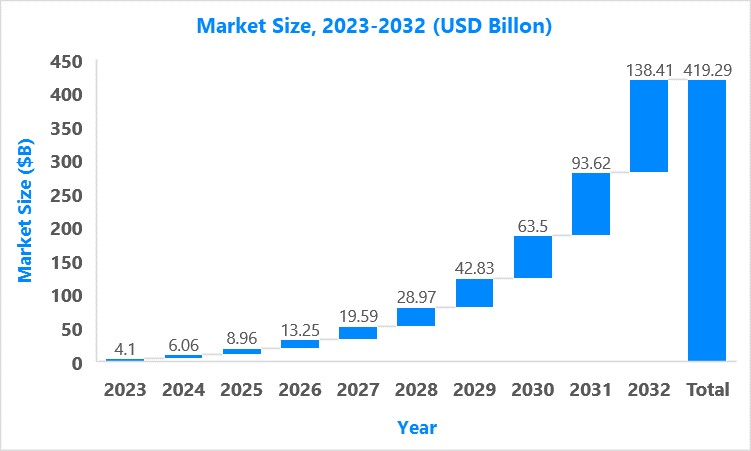
Market Size8
The global Duchenne muscular dystrophy treatment market size was valued at $4.1B in 2023 and is forecasted to reach a value of $138.41B by 2032 at a CAGR of 47.85% between 2023 and 2032.
Management and Treatment1,2,3,4,5
Currently, there is no cure for Duchenne muscular dystrophy, but there are certain medications and therapies that ease the symptoms, protect muscles, keep hearts and lungs healthy, and improve quality of life.
Several management strategies and treatments are aimed at improving symptoms, quality of life, and delaying disease progression. These may include:
- Corticosteroids
- Physical Therapy
- Occupational Therapy
- Assistive Devices
- Respiratory Support
- Cardiac Care
- Nutritional Support
- Psychosocial Support
Product Dashboard9,10
Elevidys
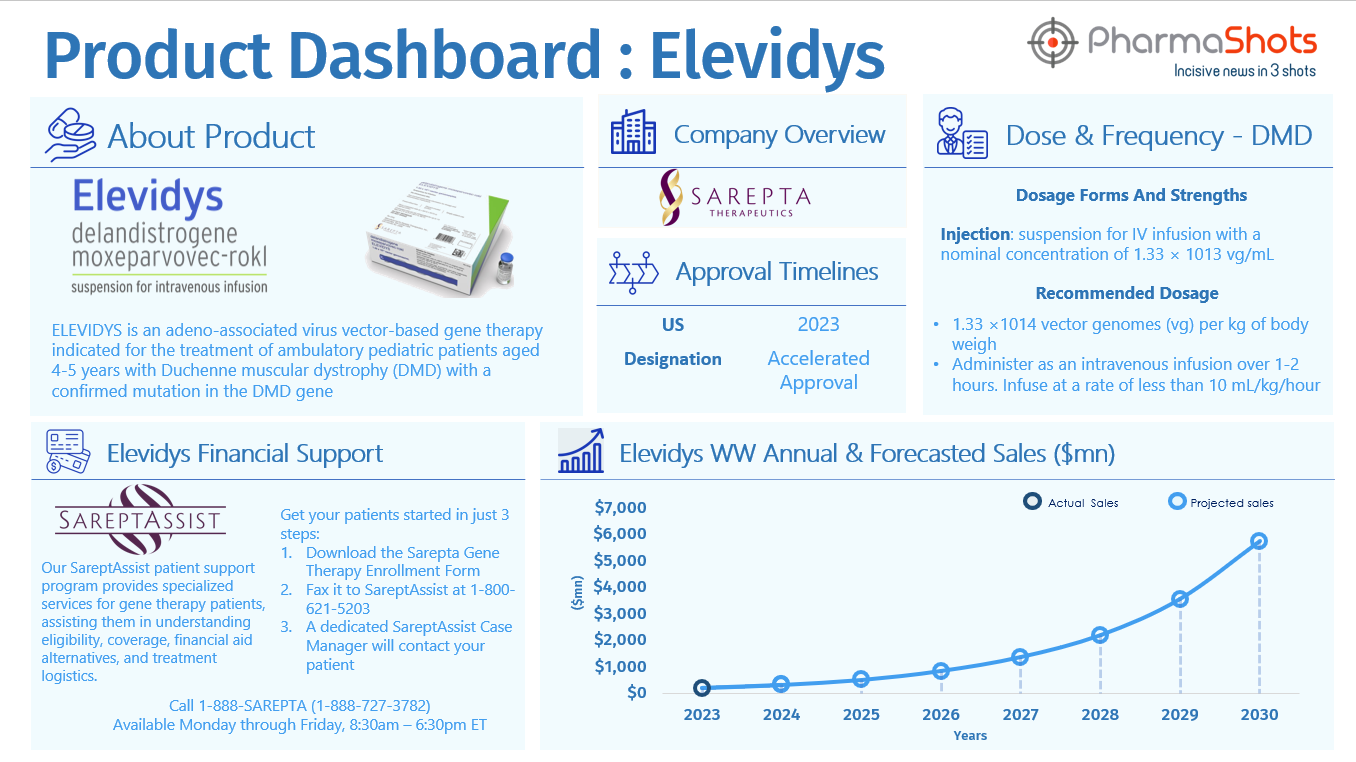
- On Dec 23, 2019, Sarepta entered into a licensing agreement with Roche for exclusive commercial rights to develop, manufacture, and commercialize ELEVIDYS (SRP-9001) in all countries outside of the U.S.; Total Deal Value: $2.850B
- On Jun 22, 2023; ELEVIDYS, the First gene therapy received FDA approval to treat Duchenne Muscular Dystrophy
- On Dec 2, 2023, Sarepta submitted a supplement BLA application to expand its label indication to remove age and ambulatory restrictions and transition its accelerated approval to traditional approval
- On Feb 16, 2024, the FDA accepted and filed Sarepta’s efficacy supplement to evaluate expanding the label by removing age and ambulation restrictions and converting the approval from accelerated to traditional
- FDA granted priority review to supplement BLA and PDUFA of Jun 21, 2024
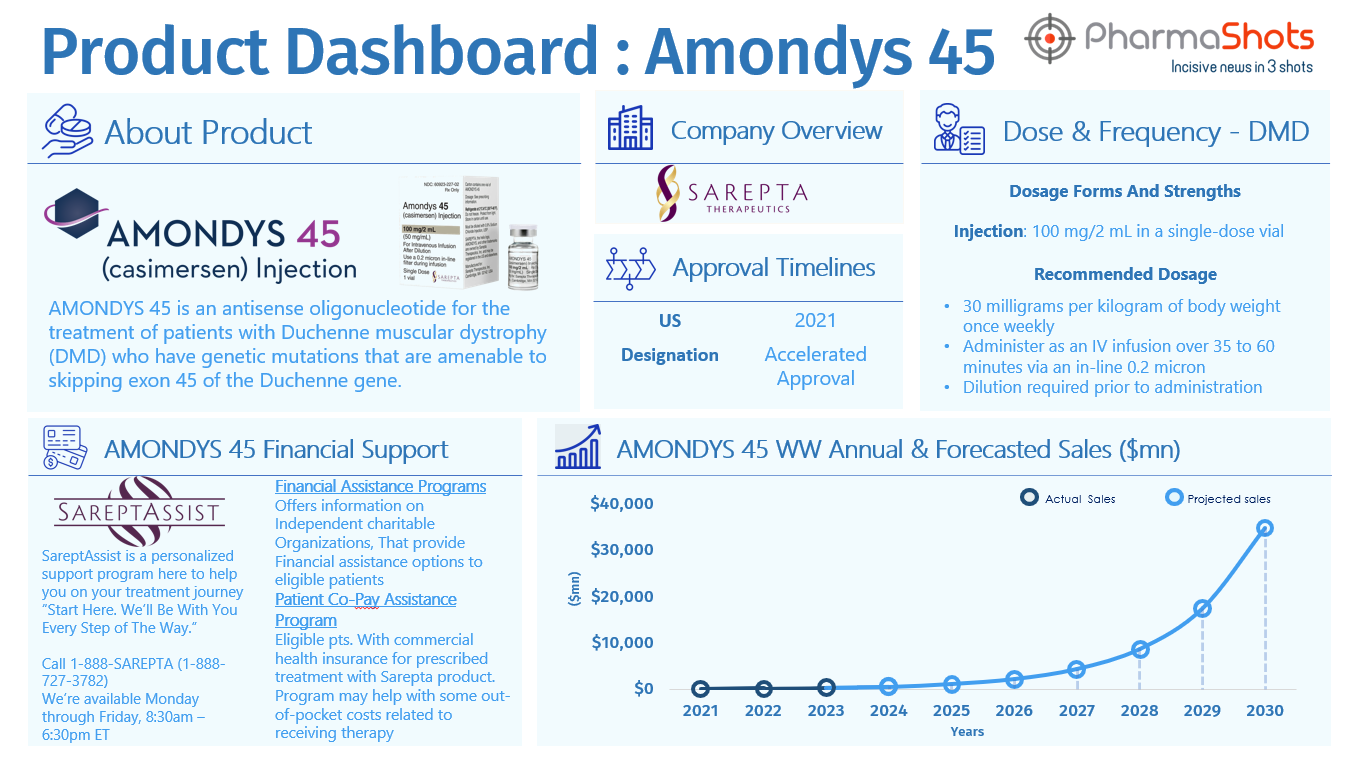
Amondys 45
- An exon 45 skipping drug, AMONDYS 45 is an antisense oligonucleotide approved for the treatment of DMD patients
- The technology binds to exon 45 of dystrophin pre-mRNA, causing exon skipping during mRNA processing. The drug has been approved under accelerated review due to increased dystrophin production in skeletal muscle
- Continued approval may be based on clinical benefits demonstrated in confirmatory trials (the ESSENCE trial), which is a placebo-controlled confirmatory trial to support its approval, which is currently ongoing and expected to conclude in 2024
Key Players in the Market11,12
Today, there are many biopharma companies working steadfastly to develop new treatment options, either as monotherapy or in combination, for Duchenne muscular dystrophy.
The table below depicts approved drugs, their formulation, year of approval, designation (if any), and the companies marketing the drugs

*In Mar 2020, Viltepso was approved in Japan for the treatment of patients with DMD who are amenable to exon 53 skipping therapy
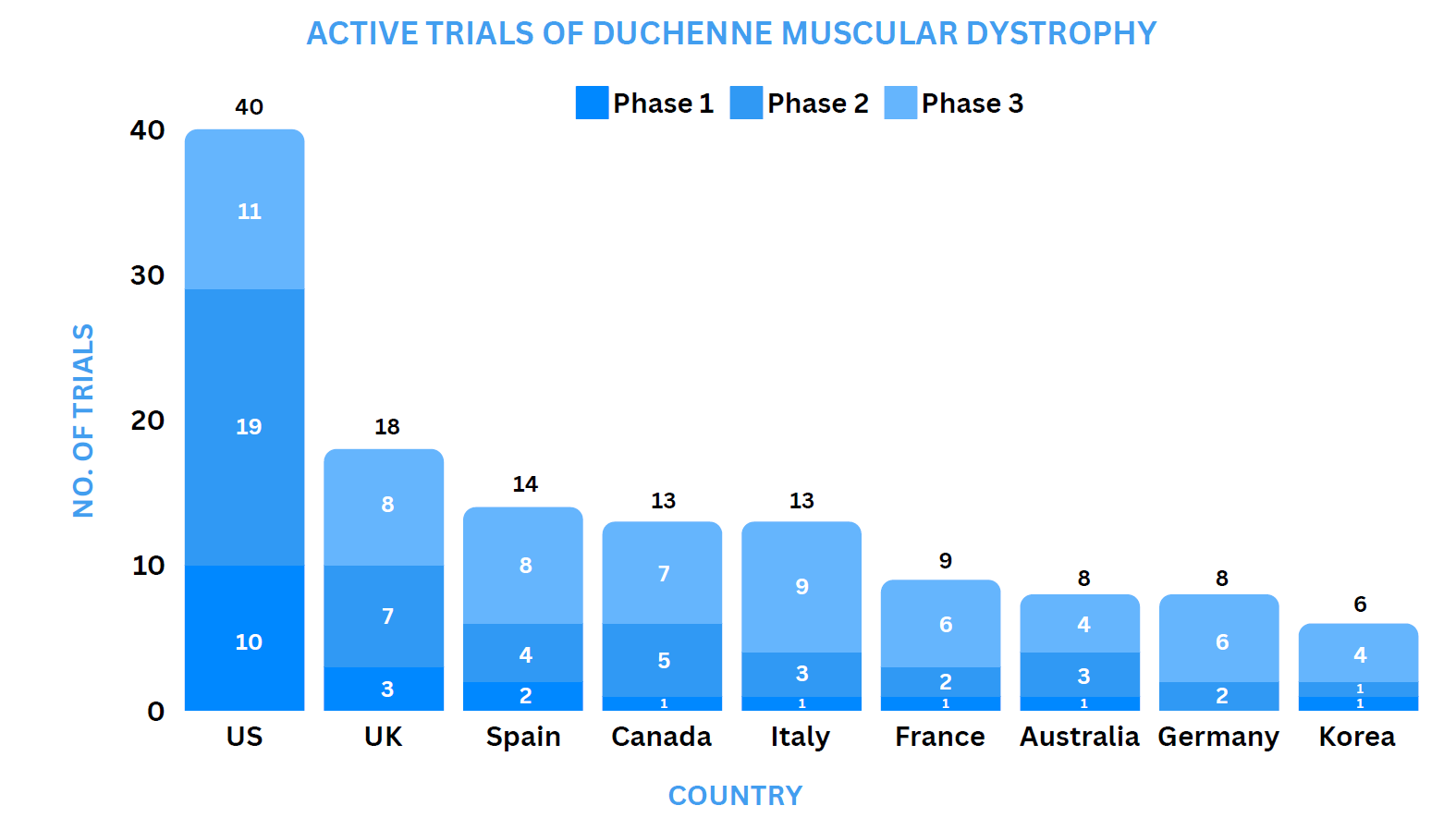
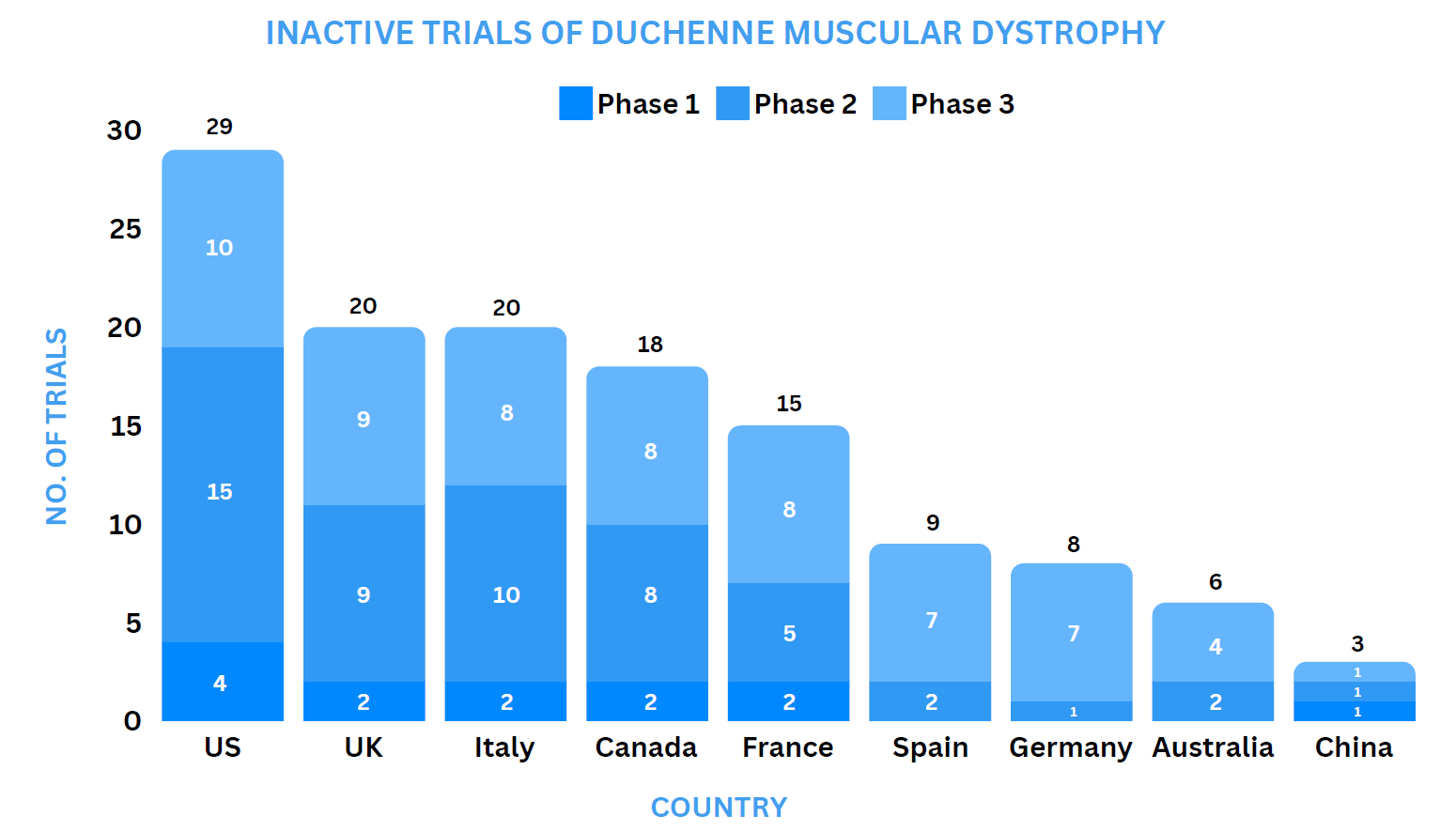
Clinical Trial Analysis13
As of April 5, 2024, about 167 interventional clinical trials have been registered worldwide for Duchenne muscular dystrophy.
Some of the key molecules involved in the trials are PF-06939926 (Pfizer), TAS-205 (Taiho Pharmaceutical), Pamrevlumab (FibroGen), CAP-1002 (Capricor).
Based on the geographical distribution, the interventional and industry-sponsored clinical trials are classified in the below-mentioned graph into two groups based on their status: Active (recruiting, active, not recruiting, not yet recruiting and enrolling by invitation, suspended) and Inactive (withdrawn, terminated, and trials with unknown status)
The maximum number of active trials is being conducted in the US, UK, Spain, Canada, Italy, France, Australia, Germany, Korea (as represented in the graph)
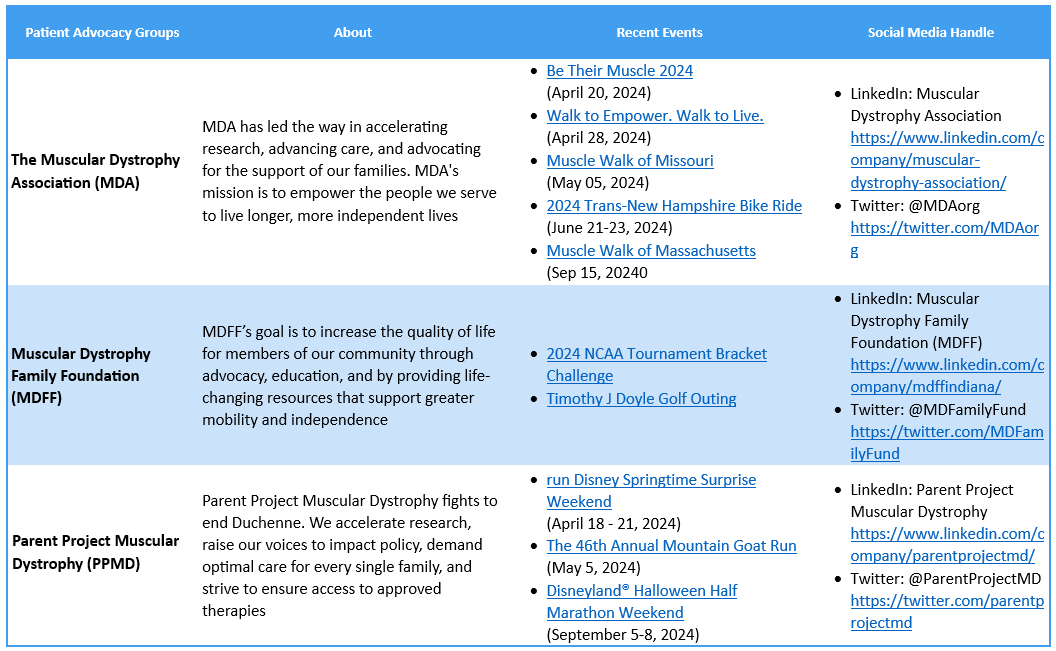
Patient Advocacy Groups (PAGs) for Duchenne Muscular Dystrophy
Patient advocacy in rare diseases like DMD can be challenging owing to low prevalence, and limited patient care. Though PAGs are dedicatedly working to address the problems of DMD patients and their families.
References
- Mayo Clinic
- Cleveland Clinic
- WebMD
- Medline plus
- Johns Hopkins Medicine
- Muscular Dystrophy Association
- Rare Disease Advisor
- Coherent Market Insight
- Elevidys
- Amondys 45
- Drugs.com
- Designation
- ClinicalTrials.Gov
Related Post: Disease of the Month - Alport Syndrome
Tags

Disha is a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.